Use Of Bacteriophages As Alternatives To Antibiotics To Treat Superbugs /Bacterial Infection In Poultry
The term ‘bacteriophage’ literally translates into “bacteria eater”. It is a type of virus that attaches itself to a bacterium and infects the host cell, subsequently destroying it. These microorganisms destroy their host cells through a natural process without interacting with human or animal cells. Since these viruses attack only bacteria; phages are harmless to people, animals, and plants. This makes the solution natural and highly safe. Researchers in different fields including medicine and animal husbandry are realizing the potential benefits of using bacteriophages in our collective war against bacterial diseases. While the use of bacteriophages in humans is still a rarity in modern medicine, their usage in animal husbandry, rearing poultry and fish is already showing positive results.
A weapon against antibiotic resistance:
Antibiotic resistance is arguably one of the biggest healthcare threats of this century. The rate of evolution of bacteria has surpassed the rate of creation of new antibiotics resulting in superbugs that are practically untreatable with the help of known antibiotics. This menace has not only made treatment of several diseases difficult but has also invaded our food systems and food chain.
It is estimated that at least 700,000 people die every year due to drug-resistant diseases, including 230,000 people who die from multidrug-resistant tuberculosis. According to a report by the UN Ad hoc Interagency Coordinating Group on Antimicrobial Resistance, as many as 10 million deaths annually could occur every year due to drug resistant diseases by 2050 if no action was taken to resist this challenge. The same report estimated that by 2030, drug resistance could push to 24 million people into poverty.
 |
| Use Of Bacteriophages as Alternatives To Antibiotics |
This is where the role of phage therapy as a possible alternative becomes important. Phages are everywhere, i.e. on human hands, skin, in the animal gut and in the soil. They are the oldest and most numerous organisms on Earth and have been protecting humans and animals from bacteria for 3.5 billion years. Phages not only eliminate selected bacteria harmlessly, they also do so without causing any damage to the gut microbiome of animals (and humans).
Bacteriophages: Ensuring Safety of Poultry Animals
One of the most important sectors where the use of bacteriophages has
emerged as very important is the poultry and aquaculture. Bacterial
diseases cause multi-billion-dollar economic losses for the livestock
industry. It is estimated that Salmonella and Campylobacter infections
that are rampant in poultry together account for 9 in 10 reported cases
of bacteria-related food poisonings globally. Diseases resulting from
Escherichia coli bacteria are another significant health concern
recognized as a major cause of morbidity and mortality in chickens.
Often, mass culling’s are necessitated to curb such infections causing
huge losses to the industry. To treat such diseases, poultry farmers are
compelled to use significant amounts of anti-microbials. Globally, 70%
of all antibiotics used are used in animal farming, and only 30% are
used directly in humans. This overuse of antibiotics leads to
antimicrobial-resistant bacteria, creating multi-tiered human health, food safety, and environmental risk.
Though Bacteriophages were first discovered more than 100 years ago but using them in an industrial animal farming environment has been difficult for two main reasons:
firstly, the ability to identify the right phages, and engineering solutions that would ensure their stability in an industrial animal farming environment.
As bacteria evolve and develop defense mechanisms, phages also adapt in response to counter the altered host systems. This ensures the bacteria do not become resistant to this approach.
With food-borne pathogens a major concern for food safety, the use of phage therapy to tackle bacterial diseases and promote food safety is expected to emerge as a viable approach on a larger scale, particularly at a time when antibiotic resistance is a significant health concern.
Bacteriophages (BPs) are viruses that can infect and kill bacteria without any negative effect on human or animal cells. For this reason, it is supposed that they can be used, alone or in combination with antibiotics, to treat bacterial infections.
Viruses of bacteria, known as bacteriophages or phages, were discovered nearly 100 years ago. Their potential as antibacterial agents was appreciated almost immediately, with the first ‘phage therapy’ trials predating Fleming’s discovery of penicillin by approximately a decade. Viruses that specifically kill bacteria, called bacteriophages, might one day help solve the growing problem of bacterial infections that are resistant to antibiotic treatment.
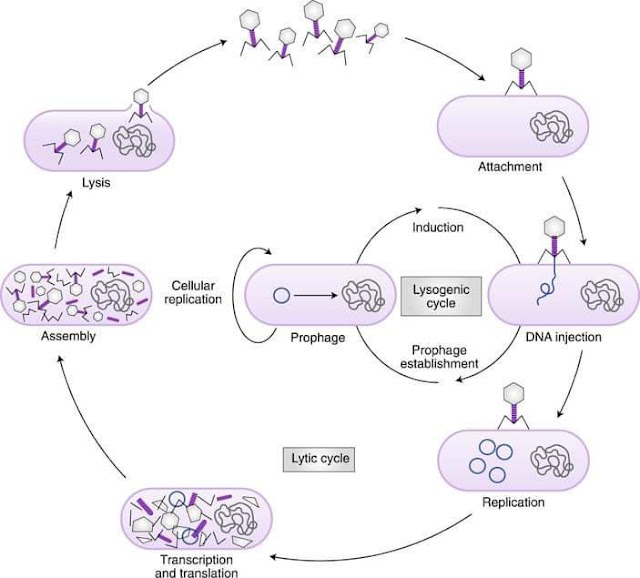
Bacteriophages are viruses that infect and kill bacteria. Bacteriophage kill bacteria, which provides an opportunity to use bacteriophage as an alternative to antibiotics to prevent and treat bacterial infections and to reduce foodborne pathogens on agricultural products. Bacteriophages are safe, having no activity against animal or plant cells, and appear to have evolved with bacteria as they are ubiquitous in nature. Bacteriophages were co discovered in the early 1900s by Twort (1915) and d’Herelle (1917). Bacteriophages are typical viruses that have a protein coat that encloses a nucleic acid, which can be DNA or RNA. There are 2 general types of bacteriophage, virulent and temperate, and they differ in life cycle. Virulent bacteriophage kill bacteria through a multiple-step process.
First they adsorb to
bacteria through recognition of specific attachment sites (receptors) on
the surface of the bacteria. Their nucleic acid (DNA or RNA) is then
injected into the bacterium. Viral replication is then achieved in the
bacteria. The bacteria are destroyed through lysis, resulting in an
average release of 50 to 200 daughter particles. Temperate bacteriophage
do not immediately replicate in the bacterium they infect, but coexist
within the bacterium as a prophage (viral nucleic acid inserted into the
bacterial genome), that is replicated along with the bacterium, thereby
converting the bacterium to a lysogenic strain. When lysogenic bacteria
are stressed, the prophage can become activated, resulting in
replication of the virus and killing of the bacteria through lysis.
Generally, virulent bacteriophage provides the greatest opportunity in
various applications to control bacteria.
Bacteriophages are a group
of viruses widely distributed in nature whose life cycle is strictly
associated with the bacterial cell. They are known as bacterial
parasites because they lack the cell structure and enzyme systems
necessary for food uptake, protein synthesis or construction of new
particles, and as incomplete organisms can only replicate in a live
cell.
Bacteriophages were discovered by Twort (1915) as unidentified
molecules that inhibit bacterial growth, but in 1917 D’Herelle was the
first to isolate and characterize phages, and he also developed the
first phage therapy against fowl typhoid induced by Salmonella
Gallinarum in chickens . Positive results of the use of bacteriophages
in fighting bacterial infections have contributed to the development of
research on the potential use of viruses that destroy bacteria in
treatment of diseases in both human and animals.
Concerns over the
consequences of bacterial resistance to antibiotics with the use of
antibiotics in animal production have led to an increase in research on
alternatives to antibiotics. Bacteriophages kill bacteria, are natural,
safe, plentiful, self replicating, self limiting, can be used to
specifically target pathogens without disruption of commensal bacteria,
and have diverse biological properties. These properties make
bacteriophages an attractive alternative to antibiotics, especially
applicable for the control of antibiotic resistant bacteria. The
efficacy of bacteriophages to prevent and treat animal diseases has been
shown in almost all production animals in both laboratory and
commercial field studies, without any adverse affects in the animals.
Although the potential of bacteriophage to control significant diseases
in animal production has been demonstrated, bacteriophage therapeutics
do not represent a replacement of antibiotics. There are some
applications in animal production systems where bacteriophage
therapeutics have an advantage over the use of antibiotics and some
applications where bacteriophage therapeutics are at a disadvantage over
the use of antibiotics.
In addition, the effectiveness of antibiotics and bacteriophage therapy can be enhanced when combined to treat animal diseases.
Increasing concerns about antimicrobial resistance in
animal and human pathogens and epidemiology of transmission of
antibiotic resistance have driven the recent search for novel
alternatives to antimicrobial drugs in humans and animals. Although
bacteriophage (phage) therapy is one such alternative, it is not novel.
Soon after their discovery by Twort (1915) and d’Herelle (1917) phages
were used to control avian typhoid caused by Salmonella gallinarum. Human
applications soon followed, and by 1930–1940phages were commonly used
therapeutics, particularly in Georgia, Russia and Poland, and also in the
USA. Interest in phage therapy declined soon following the introduction
of antibiotics, but has increased dramatically particularly with recent
research serious infections caused by antibiotic resistant pathogens
such as vancomycin-resistant Enterococci and methicillin-resistant
Staphylococcus aureus (MRSA) .
Mode of action of phages:
Phages are highly plentiful in nature and share a common ecology with their bacterial hosts. Because of their ubiquitous presence they are found in the alimentary tracts of animals and humans, and in foods, soil, water, sewage and associated environmental niches. Bacteriophages can be grouped into Lytic and Temperate phages. Lytic phages exhibits a self-replicating virulent infectious cycles that results in rapid degradation of host cell DNA, replication of phages and lysis with release of hundreds of progeny phages .In contrast Temperate phages do not lyse the host cell, incorporates phage genome into host cell genome & replicates along with host DNA as prophage. Hence there is no cell destruction, it is clear that pro-phages do not offer any therapeutic effect. At an ideal ratio of phages to susceptible host bacterial cells, phages amplify themselves by repeated cycles of replication until the host cell is eliminated or killed. This ratio is referred as Multiplicity of infection (MOI). At very high MOIs, the host may be killed by ‘lysis from without’, in which attachment of many phages to a single bacterial cell results in lysis without phage replication. At low MOI, where the numbers of target bacteria are insufficient or falls below the ‘phage proliferation threshold’ level extensive lysis of host cells takes place. In other situations, phages and susceptible bacteria may co-exist in equilibrium of a predator–prey relationship. Co-existence with relatively stable numbers of hosts and phages may reflect the emergence of phage resistant subpopulations. Another important characteristic that influences the therapeutic potential of phages is their specificity, which determines their host ranges among targeted and non-targeted bacteria.Most phages offer greater specificity than antimicrobial drugs, targeting only specificsubtypes within a species, serovar or serogroup.
 |
Phage specificity is determined largely by the interaction between binding sites on their tail fibers and one or more receptors on the cell surface of the host bacterium, which may include lipopolysaccharides, proteins, capsular polysaccharides, flagella and pili. The ideal therapeutic phage will infect multiple pathogens carrying common surface receptors. such as the Salmonella phage, Felix O1, which infects most Salmonella serovars.
Replication
of bacteriophages is similar in many ways to that of eukaryotic viruses.
Both involve adsorption, penetration, replication of nucleic acids,
formation of virions, and their release from the host cell.
Bacteriophages are specifically associated with a particular bacterial
strain and exhibit strong bactericidal activity against Gram-positive
and Gram-negative bacteria. Some phages display specific affinity for
single types of bacteria, while others have a broad range of activity.
Their specificity and range of activity is determined by the presence of
receptors located on the surface of bacterial cells, among which we can
distinguish LPS fragments, fimbriae and other surface proteins .
Their
fundamental characteristic is the presence of one type of nucleic acid
as a carrier of genetic information and a capsid built from structural
proteins. In terms of DNA structure, phages can be divided into three
groups: those containing DNA in the form of a double helix, those with a
single strand of DNA, and phages containing RNA. Most known
bacteriophages have a genome consisting of double-stranded DNA. Two
types of bacteriophages are distinguished on the basis of capsid
symmetry: isometric (polyhedral) and helical (spiral).
Phage therapy in chickens:
A number of studies have indicated the applicability of bacteriophages (both prophylactically and therapeutically) in fighting various bacterial infections of poultry viz., Escherichia coli; Salmonella enteritidis, Salmonella typhimurium and Salmonella Gallinarum; Campylobacter jejuni and Campylobacter coli; Listeria monocytogenes that are responsible for colibacillosis, salmonellosis and listeriosis, respectively and thus prevent morbidity and mortality in chickens. Two bacteriophages isolated from wastewater and poultry faecal samples, namely EC-Nid1 and EC-Nid are shown to be highly effective against O1, O2 and O78, the predominant E. coli serogroups/strains. Phages can be used to significantly reduce the caecal colonization of S. enterica serotype enteritis and typhimurium in commercial broiler chickens. Administration of 104 pfu of phage AB2 to newly hatched chicks cause decrease in the number of S. typhimurium in croup and in the small intestine and caeca respectively. Combination of bacteriophages isolated from free-range chickens may be efficacious in reducing the concentration of Salmonella enterica serovar Enteritidis phage type 4 (S. enteritidis PT4) in the caeca of broilers, therefore reducing contamination of poultry products .A multivalent bacteriophage cocktail (INT-401) has been reported to be effective for controlling necrotic enteritis of broiler chickens, caused by Clostridium perfringens with improvement in weight gain and feed conversion ratios .In birds carrying bacteriophages, the recovery of Campylobacter and the number of their strains has been reported to be reduced .Applying bacteriophages to chicken skin has revealed that a high titre of bacteriophages (107 pfu mL-1) significantly reduced the numbers of Campylobacter isolated .Phage therapy, like with two candidate phages CP8 or CP34, for C. jejuni in broiler chickens has been employed for both preventative and therapeutic purposes .Administration of bacteriophages like phage R- active and others decrease and/or eliminate E. coli infection and its various disease manifestations like septicemia, air sacculitis in birds .Large/high numbers of bacteriophages can successfully reduce the levels of Salmonella on processed poultry (broiler and turkey carcasses). Application of lytic phages has been found very effective in reducing Salmonella and Campylobacter contamination of chicken skin even resistant to antibiotics and can be helpful in providing safer poultry meat at processing and/or packaging .Listeria phages have been isolated with a wide host range, including multiple serotypes of L. monocytogenes and other Listeria spp. Bacteriophage therapy is a valuable option for controlling Listeria in undercooked poultry products. Recently, the FDA has permitted bacteriophages preparation as an anti-listerial agent for L. monocytogenes in RTE meat and poultry products and control on both raw and ready-to-eat food products .
The presence of bacterial pathogens
like Salmonella, E. coli, Listeria and Campylobacter in undercooked
poultry is implicated as the natural source of human infection, for
which bacteriophage therapy is a valuable option. Again use of phages in
combination with competitive exclusion to reduce Salmonella from
infected chicken have been investigated by developing a ‘Cocktail” of
distinct phage (i.e., phage showing different host ranges and inducing
different types of plaques on Salmonella typhimurium cultures) and
tested it in vitro as well as in vivo. Results indicate a protective
effect of Salmonella specific phages against Salmonella colonization of
experimentally infected chickens. Phages specific for various Salmonella
spp. in vitro could thus reduce the incidence of Salmonella recovery on
processed broiler and turkey carcasses and can effectively reduce the
levels of Salmonella on processed poultry .
Administering phages to
poultry via food, water or aerosol spray can be successful on a
commercial scale. Doses vary from 106 to 108 pfu mL- or higher.
Limitations:
Despite the significant positive aspects of phage therapy, there are also some limitations in the widespread use of bacteriophages to eliminate pathogens. One of the main obstacles to elimination of bacteria from poultry is that significant numbers of phages are needed to adsorb individual host cells . Some authors have shown that the application of phages in lower doses, e.g. 102 PFU, provided no statistically significant protection against E. coli infection. Moreover, preventive treatment in phage therapy did not prevent colonization
High variation in the protective dose, in some cases it was 104 PFU and some cases 1011.Steps in Lytic and lysogenic cycle of bacteriophages———– full characterization and screening of phages can eliminate those that encode toxic proteins.
To increase the safety of bacteriophages in the elimination of pathogens, the following can be recommended: the use of only strong lytic bacteriophages, not lysogenic phages, switching to non-lysing tailocins if toxic proteins released from the bacteria become a problem; the use of rapid DNA sequencing to characterize phages used in therapy; and prescreening of patients for hyper-immune reactions to the specific phage sample prior to injection, especially in whole flocks.
BACTERIOPHAGES: FEATURES AND MECHANISM OF ACTION
These are tadpole-shaped microorganisms; their genetic material (DNA or RNA) is surrounded by a protein coat, a hollow protein tail and tail fibers; based on life cycle are either lytic or temperate phages .Holin enzyme, which rapidly lyses the bacterial host cell and allows virion particles to further infect other bacterial cells during the course of multiplication and the mechanism can be exploited for therapeutic purpose .Certain lysins (especially those of phages of Gram-negative bacteria) are capable of affecting bacterial cells by a mechanism completely independent of their enzymatic activity .Temperate phages integrate their genome into host-cell DNA as a prophage and can transfer virulence and other harmful genes from one bacterium to another by transduction. Narrow range phages are restricted to maximum of two bacterial species. Bacteriophages are species specific, self-perpetuating, self-limiting and eco-friendly in nature and can survive in the gastrointestinal environment of animals and can minimize the threshold level of infectious organisms such as Salmonella, Shigella, Staphylococcus, Escherichia, Campylobacter etc. Phage isolates which are initially isolated by using B. subtilis and E. coli have also been tested for lytic activity against more bacteria viz. Staphylococcus aureus, Salmonella spp. and Proteus vulgaris, Pseudomonas aeruginosa and Klebsiella pneumoniae. Phage host range is not always genera restricted and phages could be of wide host range.
